Activity of Vinpocetine in the Cerebellum of Nickel Chloride-Exposed Rats: An in-vivo and in-silico Study
DOI:
https://doi.org/10.26538/tjdr/v2i5.4Keywords:
Nickel Chloride, Vinpocetine, Cerebellum, Caspase-3, NF-κBAbstract
Purpose: Nickel chloride (NiCl2), widely used in industry, poses neurotoxic risks, particularly to the cerebellum, which regulates motor and cognitive functions. Vinpocetine, an antioxidant and anti-inflammatory agent, may offer neuroprotection. This study investigated vinpocetine’s protective effects against NiCl2-induced cerebellar toxicity in Wistar rats.
Methods: Forty-eight (42) Wistar rats were randomly distributed to six groups (n=8) and received the following treatments. Group A (control) - 1 ml distilled water; Group B - 5 mg/kg NiCl2; Groups C and D - 2.5 mg/kg and 5 mg/kg vinpocetine, respectively, alongside 5 mg/kg NiCl2; Groups E and F - 2.5 mg/kg and 5 mg/kg vinpocetine, respectively. Treatments were administered orally for 28 days, followed by neurobehavioral, oxidative stress, histological, and in-silico assessments.
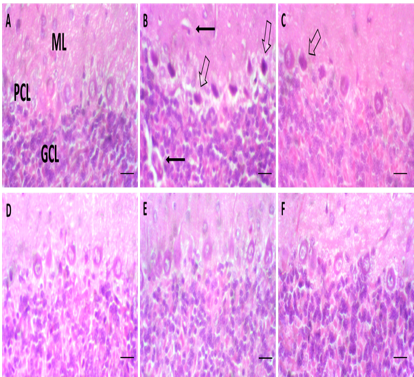
Results: NiCl2 significantly (p<0.05) reduced body, brain, and relative cerebellar weight. Behavioral deficits included impaired grip strength, coordination, and mobility. Oxidative stress markers showed reduced antioxidant enzymes activity and elevated lipid peroxidation. Histological examination revealed Purkinje cell degeneration and pyknotic nuclei in NiCl2-exposed rats. Vinpocetine treatment significantly improved motor function, antioxidant activity, and cerebellar structure while reducing oxidative damage.
Conclusion: These findings suggest that vinpocetine protects against NiCl2-induced cerebellar toxicity in Wistar rats
Downloads
References
1. Tobalu FO, Enogieru AB. Lead Neurotoxicity in Experimental Models: A Systematic Review on Effects on the Cerebrum, Cerebellum, and Hippocampus. Toxicol. Rep. 2025:102044.
2. Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health. 2020;17(3):679.
3. Ijomone OM, Olatunji SY, Owolabi JO, Naicker T, Aschner M. Nickel-induced neurodegeneration in the hippocampus, striatum and cortex; an ultrastructural insight, and the role of caspase-3 and α-synuclein. J. Trace Elem. Med. Biol. 2018;50:16-23.
4. Ijomone OM, Okori SO, Ijomone OK, Ebokaiwe AP. Sub-acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats’ brain. Drug Chem. Toxicol. 2018;41(4):377-384.
5. Liu G, Yang C, Wang X, Chen X, Cai H, Le W. Cerebellum in neurodegenerative diseases: advances, challenges, and prospects. Iscience. 2024;27(77):111194.
6. Adedara IA, Adegbosin AN, Abiola MA, Odunewu AA, Owoeye O, Owumi SE, et al. Neurobehavioural and biochemical responses associated with exposure to binary waterborne mixtures of zinc and nickel in rats. Environ. Toxicol. Pharmacol. 2020;73:103294.
7. Szatmári S, Whitehouse P. Vinpocetine for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2003;2003(1):1-17.
8. Al-Kuraishy HM, Alexiou A, Papadakis M, Elhussieny O, Saad HM, Batiha GE-S. New insights on the potential effect of vinpocetine in Parkinson’s disease: one of the neglected warden and baffling topics. Metab. Brain Dis. 2023;38(6):1831-1840.
9. Zhou Q, Guo D, Li X, Wang Y, Ye X, Xue S, Wang X. Anti-inflammatory effects of vinpocetine in LPS-stimulated microglia via activation of AMPK. An. Acad. Bras. Ciênc. 2020;92:e20200241.
10. Zhang Y-s, Li J-d, Yan C. An update on vinpocetine: new discoveries and clinical implications. Eur. J. Pharmacol. 2018;819:30-34.
11. NIH. National Institute of Health. Guide for the care and use of laboratory animals: US Department of Health and Human Services, Public Health Service, National Publication. No 85-23; 1986.
12. Enogieru A, Omoruyi S. Exploration of Aqueous Phyllanthus amarus Leaf Extract as a Protective Agent in Mercury Chloride-Exposed Wistar Rats: A Neurobehavioural Study. J. Appl. Sci. Environ. Manage. 2022;26(4):629-637.
13. Enogieru AB, Inneh CA. Cadmium and Mercury Exposure: Oxidative, Neurobehavioural and Histological Alterations to the Cerebellum of Wistar Rats. Ibom Med. J. 2022;15(2):141-7.
14. Ijomone OM, Olaibi OK, Biose IJ, Mba C, Umoren KE, Nwoha PU. Performance of motor associated behavioural tests following chronic nicotine administration. Ann. Neurosci. 2014;21(2):42.
15. Enogieru AB, Idemudia OU. Antioxidant activity and upregulation of BDNF in lead acetate–exposed rats following pretreatment with vitamin E. Comp Clin Path. 2024:1-12.
16. Enogieru AB, Iyoha EN. Role of Nitric Oxide, TNF–α and Caspase-3 in Lead Acetate-Exposed Rats Pretreated with Aqueous Rosmarinus officinalis Leaf Extract. Biol. Trace Elem. Res. 2023:1-11.
17. Guttridge J, Wilkins C. Copper dependent hydroxyl radical damage to ascorbic acid. Formation of thiobarbituric acid reactive products. FEBS lett. 1982;137:327-340.
18. Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970;34(1):30-38.
19. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170-3175.
20. Drury R, Wallington E. Carleton’s histological technique 5th ed. New York: Churchill Livingstone. 1980.
21. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455-461.
22. DeLano WL. Pymol: An open-source molecular graphics tool. CCP4 Newsl Protein Crystallogr. 2002;40(1):82-92.
23. Kaufer DI. Neurobehavioral assessment. CONTINUUM: Lifelong Learning in Neurology. 2015;21(3):597-612.
24. Yi P-L, Tsai C-H, Lu M-K, Liu H-J, Chen Y-C, Chang F-C. Interleukin-1β mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep. 2007;30(4):413-425.
25. Martínez-Martínez MI, Muñoz-Fambuena I, Cauli O. Neurotransmitters and behavioral alterations induced by nickel exposure. Endocr. Metab. Immune Disord. Drug Targets. 2020;20(7):985-891.
26. Abu-Elfotuh K, Hamdan AME, Abbas AN, Alahmre ATS, Elewa MA, Masoud RAE, Ali AA, Othman A, Kamal MM, Hassan FAM, Khalil MG, El-Sisi AM, Hady MMM, El-Azazy MKA, Awny MM, Wahid A. Evaluating the neuroprotective activities of vinpocetine, punicalagin, niacin and vitamin E against behavioural and motor disabilities of manganese-induced Parkinson's disease in Sprague Dawley rats. Biomed. Pharmacother. 2022;153:113330.
27. Althomali RH, Abbood MA, Saleh EAM, Djuraeva L, Abdullaeva BS, Habash RT, AlhassanMS, Alawady AHR, Alsaalamy AH, Najafi MLAlhassan MS, Alawady AHR, Alsaalamy AH, Najafi ML. Exposure to heavy metals and neurocognitive function in adults: a systematic review. Environ. Sci. Eur. 2024;36(1):18.
28. Ijomone OM. Neurotoxicity of nickel. Advances in neurotoxicology. 5: Elsevier; 2021. p. 263-84.
29. Halliwell B, Gutteridge JM. Free radicals in biology and medicine: Oxford University Press, USA; 2015.
23. Olufunmilayo EO, Gerke-Duncan MB, Holsinger RD. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants. 2023;12(2):517.
31. Enogieru AB, Idemudia OU. Comparative protective activity of aqueous Zingiber officinale root and Theobroma cacao seed extracts on lead acetate-induced cerebellar toxicity in rats. J. Trace Elem. Min. 2024;10:100190.
32. Enogieru AB, Olisah EC. Upregulation of caspase-3, oxidative stress, neurobehavioural and histological alterations in mercury chloride-exposed rats: role of aqueous Allium sativum bulb extract. J. Mol. Histo. 2025;56(1):1-14.
33. Ebhodaghe CI, Enogieru AB. Aluminium chloride-induced cerebral toxicity in Wistar rats: anticholinesterase and antioxidant effects of ascorbic acid. JOPAT. 2024;23(2):1548-1556.
34. D’Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. TINS. 2012;35(11):700-9.
35. Nadeem RI, Ahmed HI, El-Sayeh BM. Protective effect of vinpocetine against neurotoxicity of manganese in adult male rats. N-S Arch PharmacoL. 2018;391(7):729-742.
36. Babkina I, Sergeeva S, Gorbacheva L. The role of NF-κB in neuroinflammation. Neurochem. J. 2021;15(2):114-128.
37. Sivamaruthi BS, Raghani N, Chorawala M, Bhattacharya S, Prajapati BG, Elossaily GM, Chaiyasut C. NF-κB pathway and its inhibitors: a promising frontier in the management of Alzheimer’s disease. Biomedicines. 2023;11(9):2587.
38. Jeon K-I, Xu X, Aizawa T, Lim JH, Jono H, Kwon D-S, Abe J-I, Berk BC, Li J-D, Yan C. Vinpocetine inhibits NF-κB–dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc. Natl. Acad. Sci. 2010;107(21):9795-800.
39. Morad O, Hassan R, El-hamed D, Morad S, Abdelmageed N. Vinpocetine suppresses inflammatory and oxidative machineries in acute model of inflammation—pivotal role of COX-2 signaling. SVU- Int. J. Vet. Sci. 2021;4(3):51-69.
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.




