Fumarate ameliorated doxorubicin-induced nephrotoxicity: The role of pro-inflammatory cytokines and endothelial nitric oxide synthase signaling pathway
DOI:
https://doi.org/10.26538/tjdr/v1i1.4Keywords:
tricarboxylic acid cycle, nephrotoxicity, fumarate, endothelial nitric oxide synthase, doxorubicin, CytokineAbstract
Purpose: Nephrotoxicity is a deleterious effect of doxorubicin (dox). This study investigated the renoprotective property of the tricarboxylic acid cycle metabolite, fumarate, in dox-induced nephrotoxicity.
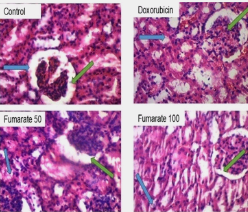
Methods: Male Wistar rats were randomly divided into four groups containing eight animals each; I: distilled water (10 ml/kg, po), II: dox (10 mg/kg stat. ip), III: dox (10 mg/kg, ip) + fumarate (50 mg/kg, po) and IV: dox (10 mg/kg, ip) + fumarate (100 mg/kg, po). The animals were treated for 10 days and euthanised on the last day. The kidneys were excised and immediately frozen for molecular analysis. A kidney section was fixed in formalin + saline solution for the histological assay.
Results: Fumarate at 50 mg/kg caused a 23. 2 %, p<0.01 reduction in kidney injury molecule (KIM) expression in dox-treated rats. There was a reduction in the expression of interleukin (IL)-1β expression in nephrotoxic rats at 100 mg/kg of fumarate, (32.4±0.6 vs 28.5±0.0, p<0.05). Similarly, IL-6 expression was decreased in a dose-dependent manner in dox-treated rats. The initial fall in superoxide dismutase (SOD) activity at 50 mg/kg of fumarate was reversed at 100 mg/kg (27.7±0.9 vs 28.6±0.4, p>0.05) in rats treated with dox. Endothelial nitric oxide synthase (eNOS) expression was significantly reduced in fumarate-treated dox rats at 50 mg/kg only, (27.1±0.7 vs 23.2±0.7, p<0.05). Histological sectioning of the kidney revealed distortions in the glomerulus of dox-treated rats and fumarate reversed these changes.
Conclusion: Data from this study show that fumarate ameliorated dox-induced nephrotoxicity by reducing eNOS and cytokine signaling.
Downloads
References
Boire A Burke K, Cox TR, Guise T, Jamal-Hanjani M, Janowitz T, Kaplan R, Lee R, Swanton C, Vander Heiden MG, Sahai E. Why do cancer patients die? Nat Rev Cancer. 2024;24(8):578-589.
Christidi, E., Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021;12(4), 339.
Dulf PL, Mocan M, Coadă CA, Dulf DV, Moldovan R, Baldea I, Farcas AD, Blendea D, Filip AG. In a murine model, doxorubicin-induced acute cardiotoxicity is associated with increased oxidative stress, autophagy, and inflammation. Naunyn-Schmiedeberg’s Arch Pharmacol.2023; 396 (6), 1105–1115.
Rawat, P.S., Jaiswal, A., Khurana, A., Bhatti, J.S., Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021 7; 139:111708
Qi, W., Boliang, W., Xiaoxi, T., Guoqiang, F., Jianbo, X., Gang, W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed Pharmacother. 2020 2;122:109547.
Afsar, T., Razak, S., Almajwal, A., Al-Disi, D. Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; Improvement by Acacia hydaspica tannin-rich ethyl acetate fraction. Saudi J Biol Sci. 2020;27(9):2251-2260.
Ikewuchi, C.C., Ifeanacho, M.O., Ikewuchi, J.C. Moderation of doxorubicin-induced nephrotoxicity in Wistar rats by aqueous leaf-extracts of Chromolaena odorata and Tridax procumbens. Porto Biomed J. 2021;11;6(1):129.
Edosuyi, O., Igbe, I., Oyekan, A. Fumarate and its downstream signaling pathways in the cardiorenal system: Recent insights and novel expositions in the etiology of hypertension. Eur J Pharmacol . 2023;961, 176186.
Hou E, Sun N, Zhang F, Zhao C, Usa K, Liang M, Tian Z. Malate and Aspartate Increase L-Arginine and Nitric Oxide and Attenuate Hypertension. Cell Rep. 2017;19 (8):1631–1639.
Czibik G, Bellahcene M, Aksentijević D, Smith AC, Mitchell SJ, Dodd MS, Kirwan J, Byrne JJ, Ludwig C, Isackson H, Yavari A, Støttrup NB, Contractor H, Cahill TJ, Sahgal N, Ball DR, Birkler RI, Hargreaves I, Tennant DA, Land J, Lygate CA, Johannsen M, Kharbanda RK, Neubauer S, Redwood C, de Cabo R, Ahmet I, Talan M, Günther UL, Robinson AJ, Viant MR, Pollard PJ, Tyler DJ, Watkins H. Fumarate Is Cardioprotective via Activation of the Nrf2 Antioxidant Pathway. Cell Metab. 2012;15 (3): 361–371
Edosuyi, O., Choi, M., Igbe, I., Oyekan, A. Fumarate exerted an antihypertensive effect and reduced kidney injury molecule (KIM)-1 expression in deoxycorticosterone acetate-salt hypertension. Clin Exp Hypertens. 2021; 43 (6): 555–564.
Olumegbon, L.T., Lawal, A.O., Oluyede, D.M., Adebimpe, M.O., Elekofehinti, O.O., I. Umar, H. Hesperetin protects against diesel exhaust particles-induced cardiovascular oxidative stress and inflammation in Wistar rats. Environ Sci and Pollut Res. 2022;29 (35): 52574–52589.
Elekofehinti, O.O., Lawal, A.O., Ejelonu, O.C., Molehin, O.R., Famusiwa, C.D. Involvement of fat mass and obesity gene (FTO) in the anti-obesity action of Annona muricata Annonaceae: in silico and in vivo studies. J Diabetes Metab Disord. 2020; 19 (1): 197–204.
Igbe, I., Edosuyi, O. Toxicity profile of aqueous extract of Cassia alata flower in Wistar rats. J Pharm Biores. 2016; 13 (2): 92-98.
Liu X, Guan Y, Xu S, Li Q, Sun Y, Han R, Jiang C. Early Predictors of Acute Kidney Injury: A Narrative Review. Kidney Blood Press Res. 2016;41 (5):680–700.
Karmakova, T.A., Sergeeva, N.S., Kanukoev, K.Yu., Alekseev, B.Ya., Kaprin, A.D. Kidney Injury Molecule 1 (KIM-1): a Multifunctional Glycoprotein and Biological Marker (Review). Sovrem Tekhnologii Med. 2021; 13 (3): 64-78.
Yamamoto, H., Ishida, Y., Zhang, S., Osako, M., Nosaka, M., Kuninaka, Y., Ishigami, A., Iwahashi, Y., Aragane, M., Matsumoto, L., Kimura, K., Kondo, T. Protective roles of thrombomodulin in cisplatin-induced nephrotoxicity through the inhibition of oxidative and endoplasmic reticulum stress. Sci Rep. 2024; 14 (1): 14004.
Boesen, E.I. Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM. Interleukin-1β, but not interleukin-6, enhances renal and systemic endothelin production in vivo. Am J of Physiol-Renal Physiol. 2008; 295 (2): 446–453.
Ighodaro, O.M., Akinloye, O.A. First-line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018; 54 (4):287–293.
Ardanaz, N., Pagano, P.J. Hydrogen peroxide as a paracrine vascular mediator: Regulation and signaling leading to dysfunction. Exp Biol Med. 2006; 231 (3): 237–251.
Förstermann, U., Münzel, T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006; 113 (13): 1708–1714.
Zhang, G., Lin, X., Shao, Y., Su, C., Tao, J., Liu, X. Berberine reduces endothelial injury and arterial stiffness in spontaneously hypertensive rats. Clin Exp Hypertens. 2020;42(3):257-265.
Wallace, K.B., Sardão, V.A., Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circulation res. 2020; 126 (7): 926–941.
Renu, K., V.G., A., P.B., T.P., Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy – An update. Euro J Pharmacol. 2018;818(2): 241–253.
Loboda YV, Korokin MV, Kuznetsov AV, Malorodova TN, Danilenko AP, Peresypkina AA, Gudyrev OS, Kop’eva OA, Pokrovskaya TG, Korokina LV,
Khruslova VN, Nazarenko VA, Avtina TV, Deikin AV, Dolzhikov AA, Puzanova TV, Danilenko LM . Novel derivative of nicotinic acid ameliorates doxorubicin-induced cardiac injury via regulation of redox homeostasis. Res Res Pharmacol. 2024; 10 (3): 85–93.
Zhang J, Sun Z, Lin N, Lu W, Huang X, Weng J, Sun S, Zhang C, Yang Q, Zhou G, Guo H, Chi J. Fucoidan from Fucus vesiculosus attenuates doxorubicin-induced acute cardiotoxicity by regulating JAK2/STAT3-mediated apoptosis and autophagy. Biomed Pharmacother. 2020;8; 130:110-534.
Qin, Q.-R., Chen, J., Hu, WL., Liu, JJ., Liu, ML., Huang, F., Hu, MJ. Association of tricarboxylic acid cycle-related metabolites with hypertension in older adults: a community-based cross-sectional study. J Human Hypertens. 2024; 11(12):1-10.
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.




