Sub-Chronic Toxicity: Biochemical, Hematological and Histopathological Effects of Picralima nitida (Apocynaeceae) Root Bark
DOI:
https://doi.org/10.26538/tjdr/v2i5.6Keywords:
Medicinal plants, Picralima nitida, Toxicity, Haematology, Histopathology, BiochemicalAbstract
Purpose: Picralima nitida, commonly called Akuamma, is a plant native to West Africa, and has been traditionally used for its medicinal properties. This study aimed to investigate the sub-chronic toxicity, with specific focus on biochemical, haematological and histopathological effects of the root bark of Picralima nitida.
Methods: Adult male Wistar rats were randomly divided into four groups: Group 1 (control) received distilled water only, Groups 2 – 4 (treatment groups) were administered 50, 100, and 200 mg/kg of P. nitida root bark methanol extract, respectively orally once daily for 28 days. On the 29th day, rats were sacrificed, blood samples were collected for biochemical and haematological analyses, while vital organs (liver, kidney, and heart) were excised and used for histopathological analysis.
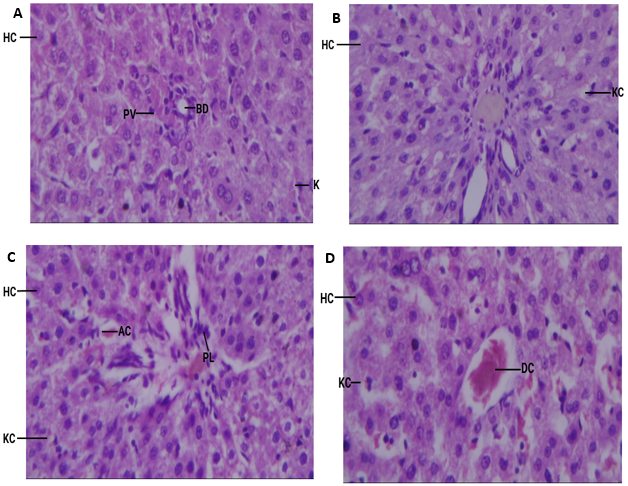
Results: Results showed that the sub-chronic administration of P. nitida root bark did not cause significant changes in serum biochemical and haematological parameters in rats. However, the extract produced significant (P<0.05) increases in mid cells (MID), mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) when compared to the control. No significant increase (P>0.05) was observed in white blood cell (WBC) count, red blood cell (RBC) count, and hemoglobin (HGB) concentration. Histopathological examination revealed normal tissue architecture, although the presence of immunological cells, and vasodilatation may indicate toxicity.
Conclusion: Findings from the study suggest that the root bark extract of P. nitida is nontoxic, and relatively safe on sub-chronic administration. However, further studies are needed with higher doses and longer duration of administration to fully understand the plant's toxicity.
Downloads
References
1. Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006; 27:1-93.
2. Shi QW, Li LG, Huo CH, Zhang Ml, Wang YF. Study on natural medicinal chemistry and new drug development. Chin Tradit Herb Drugs. 2010; 41:1593-1589.
3. Abdullahi A. Trends and Challenges of Traditional Medicine in Africa. Afr J Tradit Complement Altern Med. 2011; 8:115–123.
4. Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013; 10: 210–229.
5. Smith-Hall C, Larsen HO, Pouliot M. People, plants and health: a conceptual framework for assessing changes in medicinal plant consumption. J Ethnobiol Ethnomed. 2012; 8:43–47.
6. Nwonu C, Ilesanmi O, Agbedahunsi J, Nwonu P. Natural products as veritable surce of novel drugs and medicines: A review. Int J Herb Med. 2019; 7:50-54.
7. Hussein RA, El-Anssary AA. Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herbal Med. 2018; 1:4.
8. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999; 12:564–582.
9. Daswani GP, Brijesh S, Birdi JT. Preclinical testing of medicinal plants: advantages and approaches. Workshop Proceedings on approaches towards evaluation of medicinal plants prior to clinical trial, Foundation for Medical Research at Yashwantrao Chavan Academy of Development Administration (YASHADA), Pune. 2006; 60-77p.
10. Teugwa CM, Mejiato PC, Zofou D, Tchinda BT, Boyom FF. Antioxidant and antidiabetic profiles of two African medicinal plants: Picralima nitida (Apocynaceae) and Sonchus oleraceus (Asteraceae). BMC Complement Altern Med. 2013; 13:175–180.
11. Ojewole JAO. Acute toxicity of the aqueous root bark extract of Picralima nitida (Sterculiaceae) in mice. J Ethnopharmacol. 2000; 71:251-253.
12. Okpako EO, Oyelami OA, Ajaiyeoba EO, Adewale O, Afolabi B, Adeyemi OO. Antioxidant and antimicrobial properties of Picralima nitida (Sterculiaceae) root bark extract. J Med Plants Res. 2010; 2:215-220.
13. Akinwunmi KF, Amadi CV. Assessment of antioxidant and antidiabetic properties of Picralima nitida seed extracts. J Med Plants Res. 2019; 13:9–17.
14. Ajaiyeoba EO, Adewale O, Afolabi B, Adeyemi OO, Oluwafemi OO, Oyelami OA. Phytochemical analysis and anti-malarial activities of Picralima nitida. J Med Plant Res. 2010; 4:1348-1352.
15. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. GUIDE FOR THE CARE AND USE OF LABORATORY ANIMALS (8th Edition). Institute for Laboratory Animal Research Division on Earth and Life Studies. The National Academies Press, Washington D.C. Available from: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf
16. Jayachitra A, Rajalakshmi A, Gopal P and Krithiga N. Toxicity Analysis of different medicinal plant extracts in Swiss Albino Mice. Pharmacol Toxicol Res. 2014; 1(1):1-6.
17. Yihang L, Guang L, Meifang S, Xuelan L, Xia Z, Juan L, Xi C. Acute toxicity study of Aspidopterys obcordata aqueous extract in Sprague-Dawley rats. J Tradit Chin Med. 2016; 36(3):377-381.
18. Subramanian K, Sankaramourthy D, Gunasekaran M. Toxicity studies related to medicinal plants. In: Mandal SC, Mandal V, Konishi T, (eds). Natural products and drug discovery: An integrated approach. UK: Elsevier; 2018. 491-505p.
19. Patil UH, Gaikwad DK. Phytochemical profile and antibacterial activity of stem bark of Anogeissus latifolia. Pharmacog J. 2010; 2(17):70-73.
20. Smith AB and Jones CD. Assessing rodent health in toxicology studies: Considerations for monitoring and interpretation. J Toxicol Environ Health B Crit Rev. 2018; 21:269-289.
21. Gupta RK and Mital P. Body weight changes in toxicological studies: A critical aspect in the interpretation of toxicity data. J Toxicol Environ Health Sci. 2019; 11:1-10.
22. Awodele O, Popoola TD, Tomoye O, Afolayan G, Nwigwe I, Oyibo W (2019). Toxicological Evaluation of a Dissolvable and Disinfectant Condom in Rodents. Univ Lagos J Basic Med Sci. 2019; 7:27-30.
23. Otoo LF, Koffuor GA, Ansah C, Mensah KB, Benneh C, Ben IO. Assessment of an ethanolic seed extract of Picralima nitida ([Stapf] Th. and H. Durand) on reproductive hormones and its safety for use. J Intercult Ethnopharmacol. 2015; 4:293-301.
24. Levey AS and Coresh J. Chronic kidney disease. Lancet. 2012; 379:165-180.
25. Sharma R, Sinha R, Kaur R, Rani S. Drug-Induced Nephrotoxicity and Use of Biomarkers. In: Patel VB, Preedy VR, Rajendram R (eds) Biomarkers in Toxicology. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Cham. 2023.
26. Nwankwo NE, Egbuonu ACC, Nduka FO, Nwodo OFC. Effect of seed extract of Picralima nitida on hematological parameters of malaria-infected albino mice and its interference with the serum electrolyte levels. Ife J Sci. 2017, 19:52-57.
27. Lokwani DP. Histograms in The ABC of CBC: Interpretation of Complete Blood Count and Histograms. Jaypee Brothers Medical Publishers. 2013. 117p.
28. Bunn HF. Approach to the anemias. In: Goldman L, Schafer AI (eds) Cecil Medicine. 24th ed. Saunders Elsevier. 2011. 161p.
29. Adeneye AA, Agbaje EO, Adeyemi OO. Heamatopoietic activity of the seed aqueous extract of H. umbellate (K.schum) hallierf. In experimental anaemia. J Nat Remedies. 2010; 10:163-169.
30. Odeghe OB, Uwakwe AA, Monago CC. Some biochemical and hematological studies on the methanolic extract of Anthocleista grandioflora stem bark. Int J Appl Sci Technol. 2012; 2:58-65.
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.




