Time Course of Lead-Induced Dyslipidemia in Male Wistar Rats
DOI:
https://doi.org/10.26538/tjdr/v2i9.5Keywords:
Lead, dyslipidemia, cholesterogenesis, free fatty acids, phospholipidosis, Hydoxymethylglutaryl coenzyme A reductase.Abstract
Purpose: Previous studies have linked lead toxicity to dyslipidemia. However, this has not been well characterized in a time course study. This study investigates the effects of lead exposure on lipid metabolism with time, using a rat model.
Method: Seventy-two (72) male Wistar rats were exposed to lead at concentrations of 0, 200, 300, and 400 ppm in their drinking water for 4, 8 and 12 weeks, after which blood, liver, kidney, brain, heart, spleen and lungs were removed from the animals and analyzed for lead and lipid dynamics.
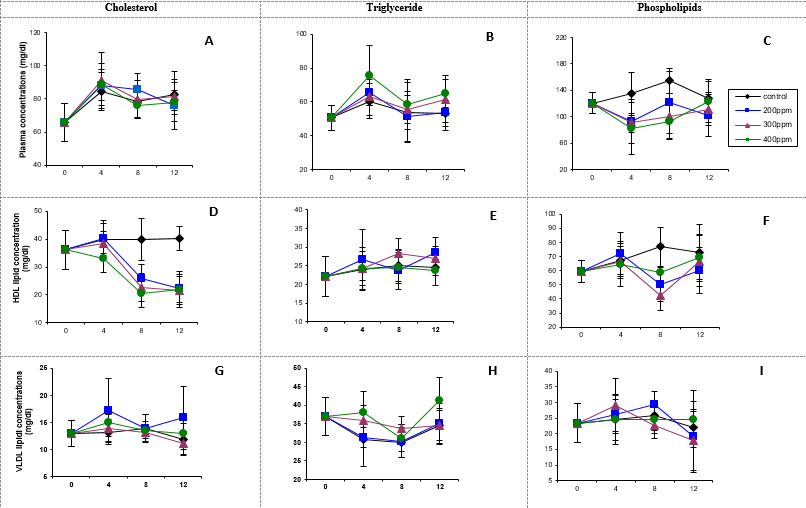
Results: Lead-induced inhibition of reverse cholesterol transport was both time-dependent as well as dose-dependent at 4 and 8 weeks. Plasma free fatty acids (FFAs) displayed a hormetic-like response at 4 weeks and increased dose-dependently at 12 weeks while erythrocyte FFAs increased in the 200 ppm dose at 4 weeks and in all the doses at 8 weeks. Increased hepatic, brain and renal cholesterogenesis were generally observed with highest increases occurring at 8 weeks in both organs. Hepatic, brain, renal, cardiac and pulmonary phospholipidosis were observed in all the lead doses and exposures. Cardiac cholesterol decreased while triglycerides increased at 4 weeks. Increases in hepatic and brain cholesterogenesis were neither dose nor time-dependent. Correlation studies showed both direct and inverse correlations between tissue lead and various lipid parameters.
Conclusion: Lead exposure induced significant dyslipidemia and altered cholesterol metabolism over time, underscoring potential cardiovascular and metabolic risks.
Downloads
References
1. Kordas K, Ravenscroft J, Cao Y, McLean EV. Lead exposure in low and middle-income countries: Perspectives and lessons on patterns, injustices, economics, and politics. Int J Environ Res. Public Health. 2018; 15(11):2351.
2. Obeng-Gyasi E. Sources of lead exposure in various countries. Reviews on Environ Health. 2019; 34(1):25-34.
3. Abam E, Okediran BS, Odukoya OO, Adamson I, Ademuyiwa O. Reversal of ionoregulatory disruptions in occupational lead exposure by vitamin C. Environ Toxicol Pharmacol. 2008; 26(3):297-304.
4. Agency for Toxic Substances and Disease Registry (ATSDR). Priority list of hazardous substances. 2011. www.atsdr.cdc.gov/spl/.
5. Kumar S, Islam R, Akash PB, Khan MHR, Proshad R, Karmoker J, MacFarlane GR. Lead (Pb) Contamination in Agricultural Products and Human Health Risk Assessment in Bangladesh. Water Air Soil Pollut. 2022; 233(7):257.
6. Gudadhe, Shubham; Singh, Sushma K.; Ahsan Jawaid. Cellular and Neurological Effects of Lead (Pb) Toxicity In: Kumar, N.; Jha, A. K. editors Lead Toxicity Mitigation: Sustainable Nexus Approaches. Environmental Contamination Remediation and Management. Springer, Cham. 2024. p. 125-145.
7. Dehari-Zeka M, Letaj KR, Selimi QI, Elezaj IR. Blood lead level (BLL), δ-aminolevulinic acid dehydratase activity (ALAD), hemoglobin (Hb) and hematocrit (hct) in primary school-children and adult residents living in smelter rural areas in Kosovo. J Environ Sci Health. 2020; 55(10):1179-1187.
8. Pukanha K, Yimthiang S, Kwanhian W. The immunotoxicity of chronic exposure to high level of lead: An Ex Vivo Investigation. J Enviro health Sci Eng. 2020;18(1):335-343.
9. Upadhyay K, Viramgami A, Bagepally BS, Balachandar R. Association between chronic lead exposure and markers of kidney injury: A systematic review and meta-analysis. Toxicol Rep. 2024; 13:101837.
10. Nakhaee S, Amirabadizadeh A, Brent J, Mehrpour O. Impact of chronic lead exposure on liver and kidney function and haematologic parameters. Basic Clin Pharmacol Toxicol. 2019; 124(5):621-628.
11. United States Environmental Protection Agency. Prevent Childhood Lead Poisoning. (2022). Available online at: https://www.epa.gov/lead/learn-about-lead (Accessed May 1, 2025).
12. Kabata-Pendias A and Szteke B. Trace elements in abiotic and biotic environments: CRC press. 2015. 465 p. doi: 10.1201/b18198
13. Kim D, Ock J, Moon K, Park C. Association between heavy metal exposure and dyslipidemia among Korean adults: From the Korean National Environmental Health Survey, 2015–2017. Int J Environ Res Public Health. 2022; 19(6):3181.
14. Zhang Y, Liu W, Zhang W, Cheng R, Tan A, Shen S, Xiong Y, Zhao L, Lei X. Association between blood lead levels and hyperlipidemiais: Results from the NHANES (1999–2018). Front Public Health. 2022; 10:981749.
15. Firoozichahak A, Rahimnejad S, Rahmani A, Parvizimehr A, Aghaei A, Rahimpoor R. Effect of occupational exposure to lead on serum levels of lipid profile and liver enzymes: An occupational cohort study. Toxicol Reports. 2022; 9:269-275.
16. Min H, Jianbin Z, Jinxia W, Peng S. Lead exposure induced lipid metabolism disorders by regulating the lipophagy process in microglia. Environ Sci Pollut Res. 2023; 30:125991-126008.
17. Obeng-Gyasi E, Armijos RX, Weigel MM, Filippelli GM, Sayegh MA. Cardiovascular-related outcomes in US adults exposed to lead. Int J Environ Res Public Health. 2018; 15:759-775.
18. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman D. Animal research: Reporting in vivo experiments: The ARRIVE Guidelines. J Cereb Blood Flow Metab. 2011; 31(4):991-993.DOI:10.1038/jcbfm.2010.220
19. Gidez LI, Miller GJ, Burstein M, Slagle S, Eder HA. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res. 1982; 23:1206-1223.
20. Folch M, Lees M, Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957; 226(1):497-509.
21. Stewart JCM. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem.1979; 104:10-14.
22. Ononogbu IC and Lewis B. Lipoprotein fractionation by a precipitation method. A simple quantitative procedure. Clin Chim Acta. 1976; 71(3):397-402.
23. Brunk SD and Swanson JR. Colorimetric method for free fatty acids in serum validated by comparison with gas chromatography. Clin Chem. 1981; 27(6):924-926.
24. Rose HG and Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res. 1965; 6:428-431.
25. Allain CC, Poon LS, Clau CSG, Richmond WY, Fu PD. Enzymatic determination of total serum cholesterol. Clin Chem. 1974; 20(4):470-478.
26. Gratzer WB. Preparation of spectrin. Methods Enzymol. 1982; 85:475-480.
27. Kriketos AD, Furler SM, Gan SK, Poyten AM, Chisholm DJ, Campbell IV, Multiple indexes of lipid availability are independently related to whole body insulin action in healthy humans. J Clin Endocr Met. 2003; 88(2):993-998.
28. Rao AV and Ramakrishnan S. Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem. 1975; 21(10):1523–1525.
29. Gonick HC. Lead-binding proteins: a review. J Toxicol. 2011; 2011:686050.
30. Nong Q, Chen B, Huang Y, Li Y, Wang Y, Liu L, He B, Luan T, Hu L, Jiang G, Identification of lead-binding proteins as carriers and potential molecular targets associated with systolic blood pressure. Chemosphere. 2023; 341:140138.
31. Shelton KR. and Egle PM. The proteins of lead-induced intranuclear inclusion bodies. J Biol Chem. 1982; 257(19):11802–11807.
32. Egle PM. and Shelton KR. Chronic lead intoxication causes a brain-specific nuclear protein to accumulate in the nuclei of cells lining kidney tubules. J Biol Chem. 1986; 261(5):2294–2298.
33. Huang Z. Association Between blood lead level with high blood pressure in US (NHANES 1999–2018). Front in Public Health. 2022; 10:836357.
34. Guo H, Tang Y, Li Y, Tian H, Zhang T, Li Y, Liu L, He B, Hu L, Jiang G. Endocytosis-mediated transport of Pb in rat blood cells. Environ Sci Technol. 2023; 57(23):8512-8523.
35. Mouniou S, Szpunor J, Lobinski R. Metallomics: the concept and methodology. Chem Rev. 2009; 109(2):2228-2248
36. Alelsandra K, Ludmita S, Michal D, Jolanta Z, Slawomir K. The effect of lead-induced oxidative stress on blood viscosity and rheological properties of erythrocytes in lead exposed humans. Clin Hemorheol Microcirc. 2014; 56(3):187-195
37. Okediran BS, Abam E, Odukoya OO, Adamson I, Ademuyiwa O. Membrane, intracellular, plasma and urinary sodium and potassium in occupational lead exposure: Effects of vitamin C supplementation. Trace Elem Electrolytes. 2009; 26(04):49-59.
38. Quintanar-Escorzar MA, Gonzalez-Martinez MT, Navarro L, Maldonado M, Arevalo B, Calderon-Salinas JV. Intracellular free calcium and transport in human erythrocytes of lead-exposed workers. Toxicol Appl Pharmacol. 2007; 220(1):1-8.
39. Babiker F, Al-Kouh A, Kilarkaje N. Lead exposure induces oxidative stress, apoptosis, and attenuates protection of cardiac myocytes against ischemia–reperfusion injury. Drug Chem Toxicol. 2018; 42(2):147–156.
40. Rajpoot A, Aggarwal T, Sharma V, Unraveling the enigma of cardiac damage caused by lead: Understanding the intricate relationship between oxidative stress and other multifactorial mechanisms. Toxicol. 2024; 509:153984.
41. Yang J, Luo J, Tian X, Zhao Y, Li Y, Wu X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants. 2024; 13(4):394.
42. Nita M and Grzybowski A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid Med Cell Longev. 2016; 2016:3164734.
43. Pirahanchi Y, Anoruo MD, Sharma S. Biochemistry, Lipoprotein Lipase. In: StatPearls, Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537040/
44. Herderson GC. Plasma free fatty acid concentration as a modifiable risk factor for metabolic disease. Nutrients. 2021; 13(8):2590.
45. Corvilain J, Loeb H, Champenois A, Abramow M. Effect of fasting levels of plasma-nonesterified fatty acids in normal children, normal adults, and obese adults. Lancet. 1961; 1:534.
46. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Raitakari OT. childhood adiposity, adult adiposity and cardiovascular risk factors. N Engl J Med. 2011; 365(20):1876-1885.
47. Calabrese JE, Osakase N, Di paola R, Siracusa R, Fusco R, D’Amico R, Impellizzeri D, Cuzzocrea S, Fritsch T, Abdelhameed SA, Wenzel U, Francesch C, Calabrese V. Hormesis defines the limits if lifespan. Ageing Res Rev. 2023; 91:102074.
48. Akano AJ, Ugbaja RN, Adenuyiwa O, Akinloye DI, Somade OT, Ojo DA, Talabi OA, Balogun EA. Gender-related alterations in free fatty Acids and oxidative stress in hypertension co-morbidly occurring with type 2 diabetes mellitus. J Nat Sci Engr Tech. 2017; 16(2):26-38
49. Adeyi OE, Babayemi DO, Ugwor EI, Adeyi AO, Afolabi AF, Popoola GA, Oyelami SO. Sub-acute exposure to sodium selenite-induced dyslipidemia, ATPase-independent electrolytes disruption and tissue damage in male Wistar rats. Afr scientist. 2020; 21(3):317-323.
50. Wirthensohn G, Gerl M, Guder WG. Triacylglycerol metabolism in isolated rat kidney cortex and outer medulla. Int J Biochem. 1980; 12(1-2):157-161.
51. Ademuyiwa O, Agarwal R, Chandra R, Behari JR. Lead-induced phospholipidosis and cholesterogenesis in rat tissue. Chem Biol Interact. 2009; 179(2-3):314-320.
52. Gu H, Territo PR, Persohn SA, Bedwell AA, Eldridge K, Speedy R, Chen Z, Zheng W, Du Y. Evaluation of chronic lead effects in the blood brain barrier system by DCE-CT. J Trace Eleme Med Biol. 2020; 62:126648.
53. Zhu Y, Wan F, Liu J, Jia Z, Song T. The Critical Role of Lipid Metabolism in Health and Diseases. Nutrients. 2024; 16(24):4414.
54. Hami J, Dashti GR, Nemat-bakhsh M, Afshar M, Ghaffari HR. The relationship between high dose lead exposure and serum lipids and lipoprotein levels. Shiraz E-Med J. 2006; 7(2):1-8.
55. Geelen MJ, Gibson DM, Rodwell VW. Hydroxymethylglutaryl-CoA reductase–the rate-limiting enzyme of cholesterol biosynthesis. A report of a meeting held at Nijenrode Castle, Breukelen, The Netherlands, August 24, 1985. FEBS Lett. 1986; 201(2):183–186.
56. Jurevics H, Hostettler J, Barrett C, Morell P, Toews AD. Diurnal and dietary-induced changes in cholesterol synthesis correlate with levels of mRNA for HMG-CoA reductase. J Lipid Res. 2000; 41:1048–1054.
57. Ravid T, Doolman R, Avner R, Harats D, Roitelman J. The ubiquitin-proteasome pathway mediates the regulated degradation of mammalian 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 2000; 275(46):35840–35
58. Omkumar RV, Darnay BG, Rodwell VW. Modulation of Syrian hamster 3-hydroxy-3-methylglutaryl-CoA reductase activity by phosphorylation. Role of serine 871. J Biol Chem. 1994; 269(9):6810–6814.
59. Brown MS and Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980; 21(5):505–517.
60. Jo Y, and Debrose-boyd RA. Post-translational regulation of HMG-CoA reductase. Cold spring harb biol. 2022; 14(12):a041253.
61. Zhang D, Wei Y, Huang Q, Chen Y, Zeng K, Yang W, Chen J, Chen J. Important hormones regulating lipid metabolism. Molecules. 2022; 27(20):7052.
62. Wu N, Sarna LK, Siow YL, Karmin O. Regulation of hepatic cholesterol biosynthesis by berberine during hyperhomocysteinemia. Am J Physiol Regul Integr Comp Physiol. 2011; 300(3):R635–R643.
63. Sato R, Goldstein JL, Brown MS. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc Natl Acad Sci USA. 1993; 90(20):9261–9265.
64. Istvan ES, Palnitkar M, Buchanan SK, Deisenhofer J. Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis. EMBO J. 200; 19(5):819–830.
65. Hofman AF and Hagey LR. Bile Acids: Chemistry, Pathochemistry, Biology, Pathobiology and therapeutics. Cell Mol Life Sci. 2008; 65(16):2461-2483.
66. Tall AR, Yvan-charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporter and cholesterol efflux: implications for treatment of atherosclerosis. Cell metabolism. 2008; 7(5):365-375.
67. Jiang XC, Bruce C, Mar J, Lin M, Ji Y, Francone OL, Tall AR. Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels. J Clin Invest. 1999; 103(6):907–914.
68. Botham KM. Lipid transport and storage. In Harper’s Illustrated Biochemistry. 27th edition. Edited by Murray RK, Granner DK, Rodwell VW. Boston: McGraw-Hill; 2006. Pp. 217–229.
69. Ali KF, Mohamed A, Hassan MAA. Protective effect of curcumin on lipid profile in rats intoxicated by cyclophosphamide. Egypt Acad J Biol Sci. 2021; 12(1):183-188.
70. Abe A, Hiraoka M, Shayman JA. A role for lysosomal phospholipase A2 in drug induced phospholipidosis. Drug Metabol Lett. 2007; 1(1):49–53.
71. Senault C, Yazbeck J, Goubern M, Portet R, Vincent M, Gallay J. Relation between membrane phospholipid composition, fluidity and function in mitochondria of rat brown adipose tissue. Effect of thermal adaptation and essential fatty acid deficiency. Biochim Biophys Acta Biomembr. 1990; 1023:283–289.
72. Wusu AD, Ogunrinola OO, Afolabi OK, Abam EO, Babayemi DO, Dosumu OA, Onunkwor OB, Balogun EA, Odukoya OO, Ademuyiwa O. Sexual dimorphism in inorganic mercury toxicokinetics and the attendant lipotoxic and non-lipotoxic dyslipidemia in the rat. Biochem Biophys Rep. 2021; 28:101146.
73. Dike SC, Anita AM, Babatunde BB, Azejiofor AN and Sikoki FD. Cognitive, sensory and motor impairments associated with aluminium, manganese, mercury and lead exposure in the onset of neurodegeneration. IPS J Public Health. 2023; 2(1):1-17.
74. Smith DR, and Strupp BJ. Animal models childhood exposure to lead or manganese: evidence for impaired attention, impulse control, and affect regulation and assessment of potential therapies. Neurotherapeutics. 2023; 20(1):3-21.
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.




