Chemical Profiling, In-vitro and Computational analysis of the Ethanol Extract of Mangifera indica Bark in the search for new antidiabetic agents
DOI:
https://doi.org/10.26538/tjdr/v2i1.3Keywords:
Mangifera indica, Mangiferin, α-glucosidase, α-amylase, Diabetes, IC50, Druglikeness.Abstract
Purpose: Diabetes mellitus is a global health challenge requiring novel therapeutic agents. The inhibition of α-amylase and α-glucosidase enzymes is a key strategy for controlling postprandial hyperglycemia. While synthetic inhibitors exist, their adverse effects necessitate safer alternatives. This study evaluates the chemical composition and antidiabetic potential of Mangifera indica bark extract through in vitro and computational analyses.
Methods: High-Performance Liquid Chromatography (HPLC) and Gas Chromatography-Mass Spectrometry (GC-MS) were used to identify phytochemicals in the extract. The α-amylase and α-glucosidase inhibitory activities were assessed using enzyme inhibition assays, with IC50 values determined. Molecular docking studies were conducted using AutoDock Vina to evaluate the binding affinity of key phytoconstituents, and pharmacokinetic properties were analyzed using SwissADME.
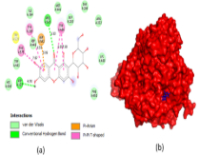
Results: HPLC and GC-MS identified gallic acid (0.58 g/g) and Mangiferin (0.03 g/g) as major bioactive compounds. The extract exhibited strong α-amylase inhibition (IC50 = 16.11 µg/mL) and α-glucosidase inhibition (IC50 = 6.96 µg/mL), outperforming Acarbose. Molecular docking revealed Mangiferin as the primary bioactive compound, with binding affinities of -9.1 kcal/mol and -7.8 kcal/mol for α-glucosidase and α-amylase, respectively. ADME analysis indicated favorable pharmacokinetics and drug-likeness properties.
Conclusion: Mangifera indica bark extract demonstrated potent antidiabetic activity through enzyme inhibition, with Mangiferin identified as a promising lead compound. These findings support its potential as a natural therapeutic agent for diabetes management, warranting further pharmacological and clinical investigations.
Downloads
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019; 157:107843. doi: 10.1016/j.diabres.2019.107843.
2. Zimmet P, Alberti KG, Magliano DJ. Diabetes in 2015: Global estimates of the prevalence of diabetes and its burden. Diabetes Res Clin Pract. 2016; 117: 123-125. doi: 10.1016/j.diabres.2016.01.031.
3. Zakir M, Ahuja N, Surksha MA, Sachdev R, Kalariya Y, Nasir M, Kashif M, Shahzeen F, Tayyab A, Khan MSM, Junejo M, Manoj Kumar F, Varrassi G, Kumar S, Khatri M, Mohamad T. Cardiovascular Complications of Diabetes: From Microvascular to Macrovascular Pathways. Cureus. 2023; 15(9):e45835. doi: 10.7759/cureus.45835.
4. Park J, Zhang P, Wang Y, Zhou X, Look K, Bigman E. Diabetes management with phytochemicals: Bioactive plant compounds to reverse insulin resistance and glucose imbalance. Sci Rep. 2022; 12: 4159.
5. Guan H, Tian J, Wang Y, Niu P, Zhang Y, Zhang Y, Fang X, Miao R, Yin R, Tong X. Advances in secondary prevention mechanisms of macrovascular complications in type 2 diabetes mellitus patients: a comprehensive review. Eur J Med Res. 2024; 29:152.
6. Salm S, Rutz J, Van den Akker M, Blaheta RA, Bachmeier BE. Current state of research on the clinical benefits of herbal medicines for non-life-threatening ailments. Front Pharmacol. 2023; 14. doi 10.3389/fphar.2023.1234701
7. Deependra Y, Kulveer SY, Singh SP. Mango: Taxonomy and botany. J. Pharmacog Phytochem. 2018; 7(2):3253-3258. Available from: https://www.phytojournal.com/archives/2018.v7.i2.4027/mango-taxonomy-and-botany
8. Gupta R, Verma S, Kumar P. Antidiabetic activity of Mangifera indica bark extract in alloxan-induced diabetic rats. Pharmacognosy Res. 2012; 4(3):162-165. doi: 10.4103/0974-8490.100396.
9. Binu P, Abhilash S, Vinetha RC, Arathi P, Harikumaran NR. Evaluation of antioxidant potential of natural xanthone mangiferin in myocardium: A rat model study. Int J. Adv Res Biol Sci. 2016; 3(11):220-229. doi: 10.22192/ijarbs.2016.03.11.028.
10. Coelho E, Souza M, Corrêa L, Viana A, Azevêdo L. Bioactive Compounds and Antioxidant Activity of Mango Peel Liqueurs (Mangifera indica L.) Produced by Different Methods of Maceration. Antioxidants. 2019; 8. 102. 10.3390/antiox8040102.
11. Ali BA, Alfa AA, Tijani KB, Idris ET, Unoyiza US, Junaidu Y. Nutritional health benefits and bioactive compounds of Mangifera indica L. (Mango) leaves methanolic extracts. Asian Plant Res J. 2020; 6(2):41-51. doi: 10.9734/aprj/2020/v6i230126.
12. Akhter S. Low to no cost remedies for the management of diabetes mellitus: Global health concern. J. Diabetes Metab Disord. 2021;20(1):951-962. doi: 10.1007/s40200-021-00783-6.
13. Aderonke AA, Ejiofor J, Eniola OW, Akanji MA, Olatunbosun OO. Evaluation of the Safety Profile of Ethanol Stem Bark Extract of Mangifera indica on Reproductive Functions of Male Albino Rats. NIJOPHASR. 2020 Aug. 16;8 (2):44-8. Available from: https://www.nijophasr.net/index.php/nijophasr/article/view/289
14. Zhang X, Su B, Li J, Li Y, Lu D, Zhu K, Zhao M. Analysis by RP-HPLC of Mangiferin component correlation between medicinal Loranthus and their mango host trees. J. Chromatogr Sci. 2012; 52(1):1–4. doi: 10.1093/chromsci/bms196.
15. Worthington V, editor. Alpha amylase. In: Worthington enzyme manual. Freehold, NJ: Worthington Biochemical Corp; 1993. p. 36-41.
16. Selin B, Irfan T. Production of β-mannanase, inulinase, and oligosaccharides from coffee wastes and extracts. Int J. Biol Macromol. 2024; 261:129798. doi: 10.1016/j.ijbiomac.2024.129798.
17. Matsui TC, Yoshimoto C, Osajima K, Oki T, Osajima Y. In vitro survey of α-glucosidase inhibitory food components. Biosci Biotechnol Biochem. 1996; 60(12):2019-2022.
18. Bräunlich M, Slimestad R, Wangensteen H, Brede C, Malterud KE, Barsett H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients. 2013; 5(3):663-678. doi: 10.3390/nu5030663.
19. Burley SK, Berman HM, Bhikadiya C, Bi C, Chen L, Di Costanzo Z, Zardecki C. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2018; 47(D1):D464–D474. doi: 10.1093/nar/gky1004.
20. Biovia DS, Systèmes D. Discovery Studio Modeling Environment. Version 53. Dassault Systèmes; 2016.
21. Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput Chem. 2010; 31(2):455-461. doi: 10.1002/jcc.21334.
22. Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Bryant SH. PubChem Substance and Compound databases. Nucleic Acids Res. 2015; 44(D1):D1202–D1213. doi: 10.1093/nar/gkv951.
23. O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: An open chemical toolbox. J. Cheminform. 2011; 3(1):33. doi: 10.1186/1758-2946-3-33.
24. Schrödinger L, DeLano W. PyMOL. The PyMOL Molecular Graphics System, Version 2. Schrödinger, LLC: New York, NY, USA; 2020.
25. Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017; 7:42717. doi: 10.1038/srep42717.
26. López-Blanco JR, Aliaga JI, Quintana-Ortí ES, Chacón P. iMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014; 42(W1):W271–W276. doi: 10.1093/nar/gku339.
27. Ning Z, Lu C, Zhang Y, Zhao S, Liu B, Xu X, Liu Y. Application of plant metabonomics in quality assessment for large-scale production of traditional Chinese medicine. Planta Med. 2013; 79(11):897-908.
28. Loan NTT, Long DT, Yen PND, Hanh TTT, Pham TN, Pham DTN. Purification process of mangiferin from Mangifera indica L. leaves and evaluation of its bioactivities. Processes. 2021; 9(5):852. doi: 10.3390/pr9050852.
29. Purushoth PT, Panneerselvam P, Suresh R, Clement AW, Balasubramanian S. GC-MS analysis of ethanolic extract of Canthium parviflorum Lamk leaf. J. Appl Pharm Sci. 2013;3(2):166-168.
30. Guo X, Jiang X, Chen K, Liang Q, Zhang S, Zheng J, Ma X, Jiang H, Wu H, Tong Q. The Role of Palmitoleic Acid in Regulating Hepatic Gluconeogenesis through SIRT3 in Obese Mice. Nutrients. 2022; 14(7):1482. https://doi.org/10.3390/nu14071482
31.Abhijit S, Rashmi S, Srivastava NS, Yashwant M, Bhagyashri C. Anti-diabetic activity of Tridax procumbens. J. Sci Innov Res. 2014; 3(2):221-226.
32. Raman BV, Naga Vamsi Krishna A, Narasimha Rao B, Pardha Saradhi M, Basaveswara Rao MV. Plants with antidiabetic activities and their medicinal values. Int Res J. Pharm. 2012; 3(3):11-15.
33. Sekar V, Chakraborty S, Mani S, Sali VK, & Vasanthi HR. Mangiferin from Mangifera indica fruits reduces post-prandial glucose level by inhibiting α-glucosidase and α-amylase activity. Sou. Afr, J. Bot. 2019; 120:129-134.
34. Ghosh P, Bhakta S, Bhattacharya M, Sharma AR, Sharma G, Lee SS, Chakraborty C. A novel multi‐epitopic peptide vaccine candidate against Helicobacter pylori: In‐silico identification, design, cloning and validation through molecular dynamics. Int J. Pept Res Ther. 2021; 27:1149–1166.
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.




